We have developed hundreds of resources to support healthcare professionals and their patients. All are free to download, print, and share in your practice. Each printable (PDF) resource includes in its footer the date it was last reviewed and a QR code that links to the current online version.
Using the Clinical Resources Library
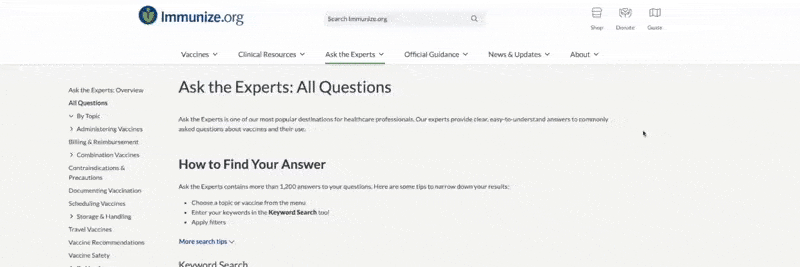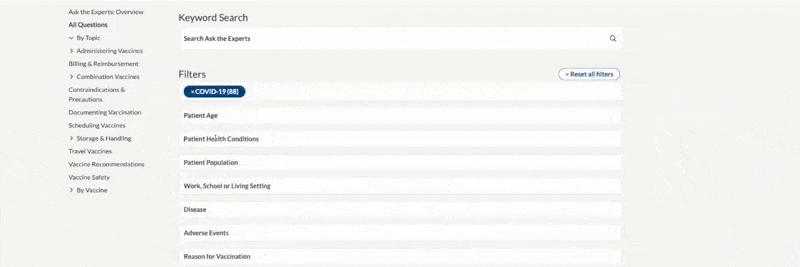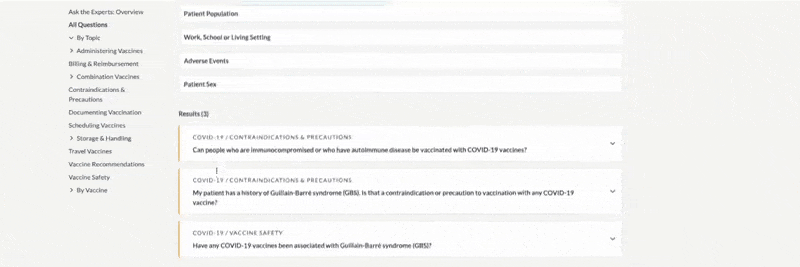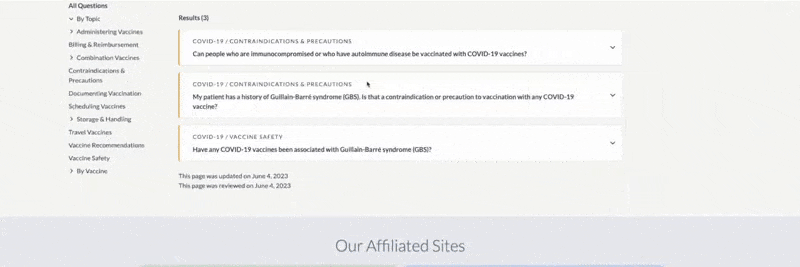
How to Find Your Answer
- By default, results are sorted alphabetically by title. You can also sort results by document number or by date (most recently updated first).
- Some filter options may become disabled as you narrow your selections.
- Filters are easily added and removed. Click or tap on the X to remove individual filters or choose “Reset All Filters” to remove all filters.