Clinical Resources
Important information about mpox vaccination
Jynneos vaccine is recommended for adults age 18 years and older at risk for mpox. See the current CDC Recommended Adult Immunization Schedule for details.
FDA Emergency Use Authorization (EUA) permits the use of Jynneos vaccine for children younger than age 18 years determined to be at high risk for mpox.
The preferred route of administration for Jynneos vaccine is subcutaneous.
Materials for Providers
Alphabetical by Title
Standing Orders for Administering Jynneos (Mpox) Vaccine by Subcutaneous Injection to Persons 18 Years of Age and Older [CDC]
Eligible healthcare professionals may vaccinate persons who meet the criteria identified on the CDC’s form.
Ask the Experts
CDC · FDA · State
ACIP Recommendations
Current Recommendations
Additional Federal Resources
ACIP
General Mpox Information
- Main page: Mpox (CDC)
- Healthcare professional page: Mpox Information for Healthcare Professionals (CDC)
CDC Mpox Vaccination Guidance
- Vaccine for Mpox Prevention in the United States (CDC)
Mpox vaccination overview - Interim Clinical Considerations for Use of Vaccine for Mpox Prevention in the United States (CDC)
Primary site for clinical mpox vaccination information - [Archive] Interim Clinical Considerations for Use of Vaccine for Mpox Prevention in the United States (archive.org)
This link goes to the archived content of the page as it appeared on the CDC website on November 25, 2024
Vaccine Administration, Storage and Handling
- Mpox Vaccine Administration Errors and Deviations (CDC)
- Vaccine Storage and Handling Toolkit: Mpox Vaccines Addendum (CDC)
- General Best Practice Guidelines for Immunization (CDC)
General information, not specific to mpox
Additional Resources
- Mpox Vaccine Locator (CDC)
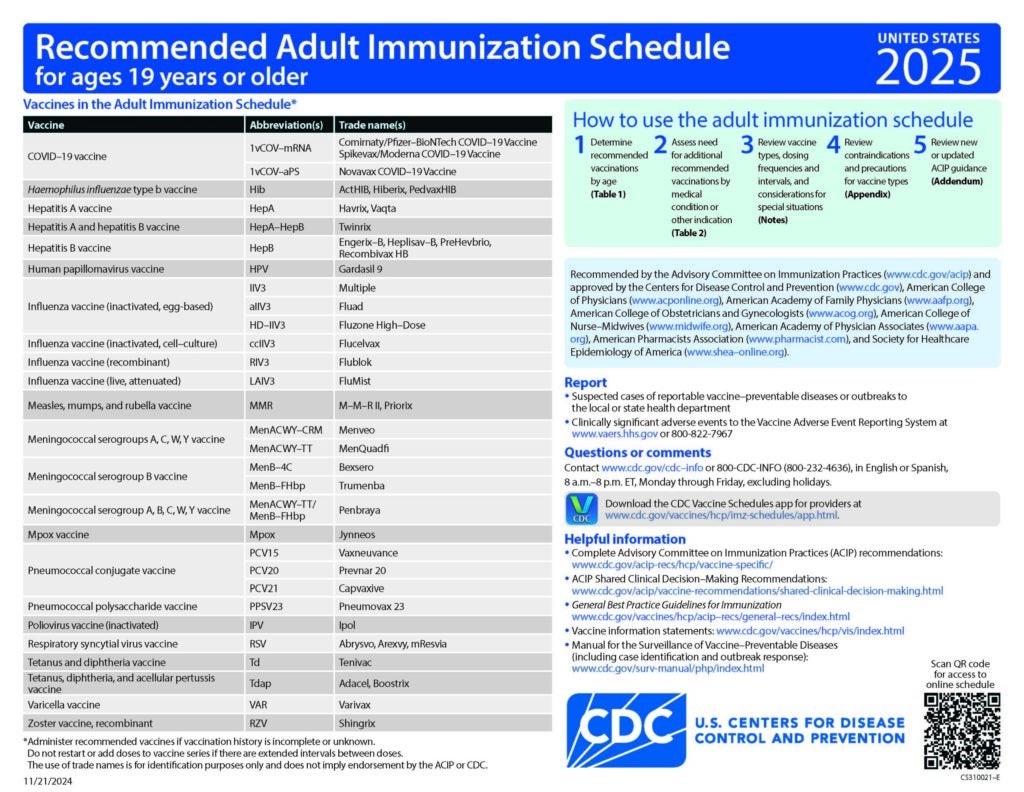
CDC Recommended Schedules
FDA Package Inserts & EUAs
Healthcare Professional Organizations
Travel
All travelers should be up to date on routine vaccines. Depending on the destination, itinerary, and duration of travel, additional vaccines may be recommended.
CDC Resources
Includes information on mpox virus
Mpox travel information