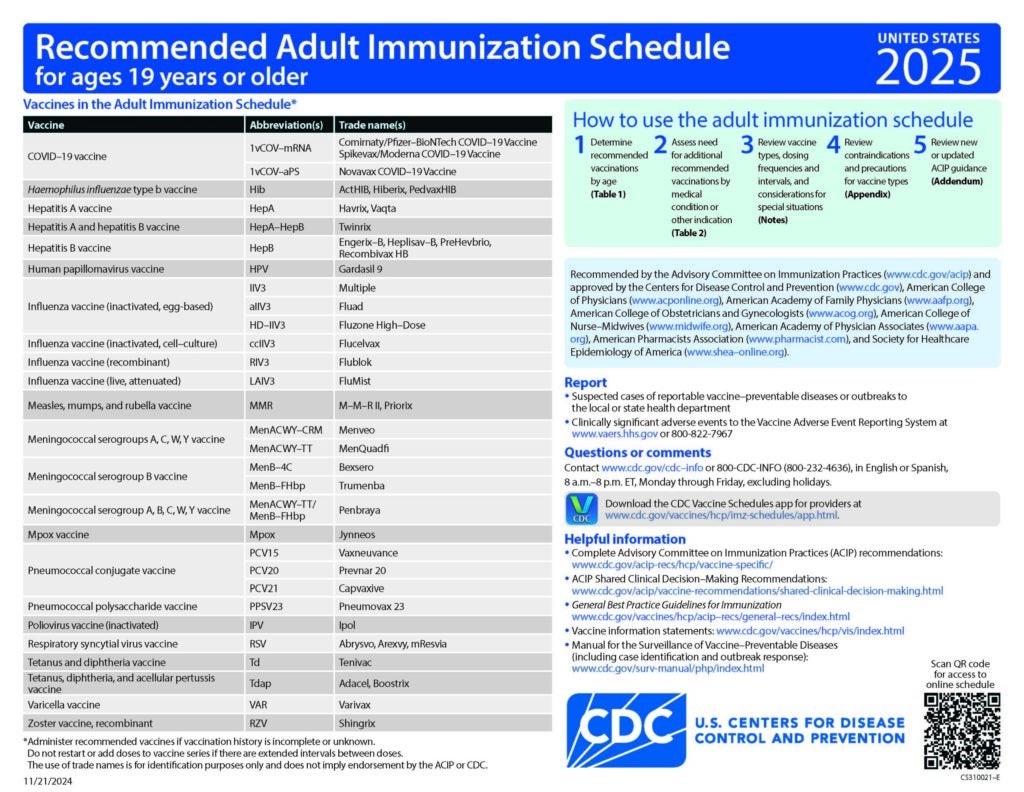
Clinical Resources
Materials for Providers
Standing Orders for Administering Nirsevimab RSV Preventive Antibody (Beyfortus, by Sanofi) to Infants and High-Risk Young Children
Eligible healthcare professionals may vaccinate infants or high-risk young children who meet any of the criteria on this form. This template is consistent with the American Academy of Pediatrics 2026 Recommended Child and Adolescent Immunization Schedule.
Standing Orders for Administering Respiratory Syncytial Virus Vaccine (RSV) to Adults Age 50 Years and Older
Eligible healthcare professionals may vaccinate adults age 50 years and older who meet any of the criteria on this form
Standing Orders for Administering Clesrovimab RSV Preventive Antibody (Enflonsia, by Merck) to Infants
Eligible healthcare professionals may vaccinate infants who meet any of the criteria on this form. This template is consistent with the American Academy of Pediatrics 2026 Recommended Child and Adolescent Immunization Schedule.
Standing Orders for Administering Pfizer Respiratory Syncytial Virus (RSV) Vaccine (Abrysvo) During Pregnancy
Eligible healthcare professionals may vaccinate all pregnant women who meet any of the criteria on this form. This template is consistent with recommendations of the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists.
CDC · FDA · State
ACIP Recommendations
Current Recommendations
Additional Federal Resources
- All current and archived ACIP RSV (Respiratory Syncytial Virus) recommendations
- General Best Practice Guidelines for Immunization
- ACIP RSV (Respiratory Syncytial Virus) recommendations at CDC
- CDC RSV (Respiratory Syncytial Virus) Information for Healthcare Professionals
- RSV Vaccination for Adults 60 Years and Older