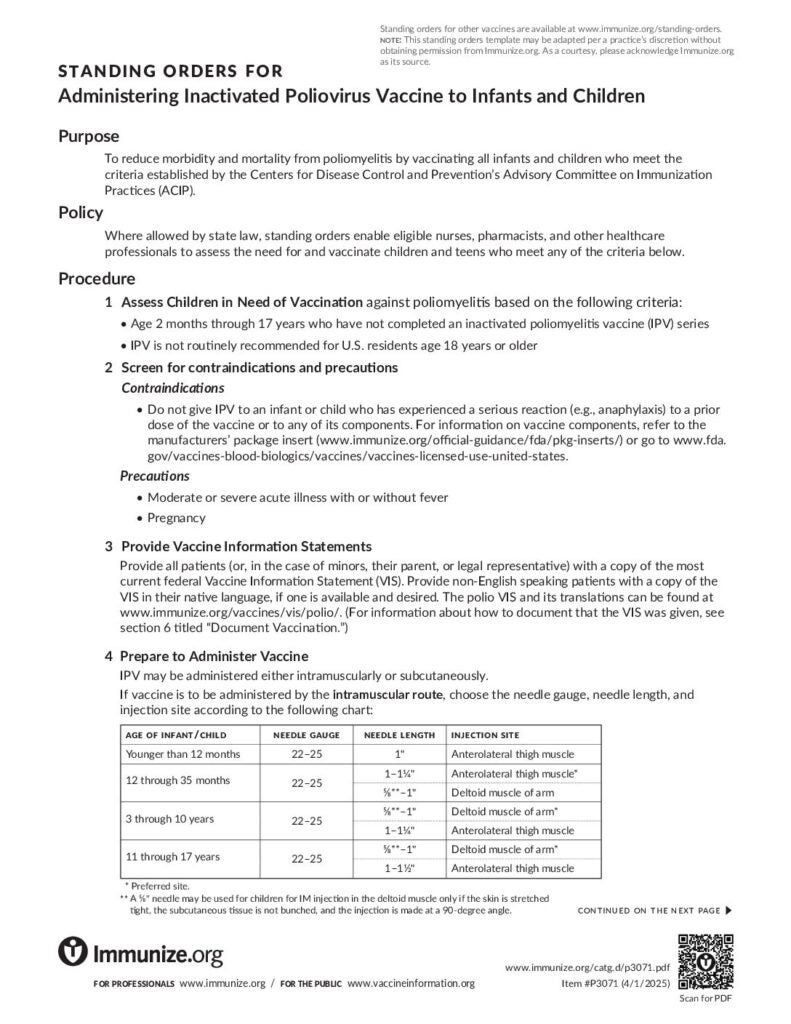
Clinical Resources
Ask the Experts
CDC · FDA · State
ACIP Recommendations
Current Recommendations
Use of Inactivated Polio Vaccine Among U.S. Adults: Updated Recommendations of the Advisory Committee on Immunization Practices — United States, 2023
MMWR December 8, 2023 / 72(49);1327–1330
Licensure of a Diphtheria and Tetanus Toxoids and Acellular Pertussis, Inactivated Poliovirus, Haemophilus influenzae Type b Conjugate, and Hepatitis B Vaccine, and Guidance for Use in Infants
MMWR, February 7, 2020, 69 (5);136-139
Guidance for Assessment of Poliovirus Vaccination Status and Vaccination of Children Who Have Received Poliovirus Vaccine Outside the United States
MMWR, January 13, 2017; 66(01); 23–25
Updated Recommendations of the Advisory Committee on Immunization Practices (ACIP) Regarding Routine Poliovirus Vaccination
MMWR, August 7, 2009; 58(30):829-30
Poliomyelitis Prevention in the United States
MMWR May 19, 2000 / Vol. 49 / No. RR-05
CDC Recommended Schedules
FDA Package Inserts & EUAs
Polio: IPOL Package Insert
Sanofi U.S.
Combination Vaccine: Vaxelis Package Insert
MSP Vaccine Company
Combination Vaccine: Kinrix Package Insert
GSK
Combination Vaccine: Pediarix Package Insert
GSK
Combination Vaccine: Pentacel Package Insert
Sanofi U.S.
Combination Vaccine: Quadracel Package Insert
Sanofi U.S.
State Policies
Travel
All travelers should be up to date on routine vaccines. Depending on the destination, itinerary, and duration of travel, additional vaccines may be recommended.
CDC Resources
Travelers’ health information for healthcare providers
Polio-specific information for travelers
Partner Resources
Nongovernmental
World Health Organization factsheet including key facts on global polio eradication
Partnership of WHO, Rotary International, CDC, UNICEF, Bill and Melinda Gates Foundation, and Gavi, The Vaccine Alliance – established to eradicate polio worldwide