Currently, the United States has the safest, most effective vaccine supply in its history. A long-standing vaccine safety system through the FDA ensures that U.S.-approved vaccines are safe, effective, and available.
Immunize.org
Materials for Vaccine Recipients
Clear Answers and Smart Advice About Your Baby’s Shots
Written by Dr. Ari Brown, clear answers to parents’ questions about vaccines.
- Also available in:
- Russian
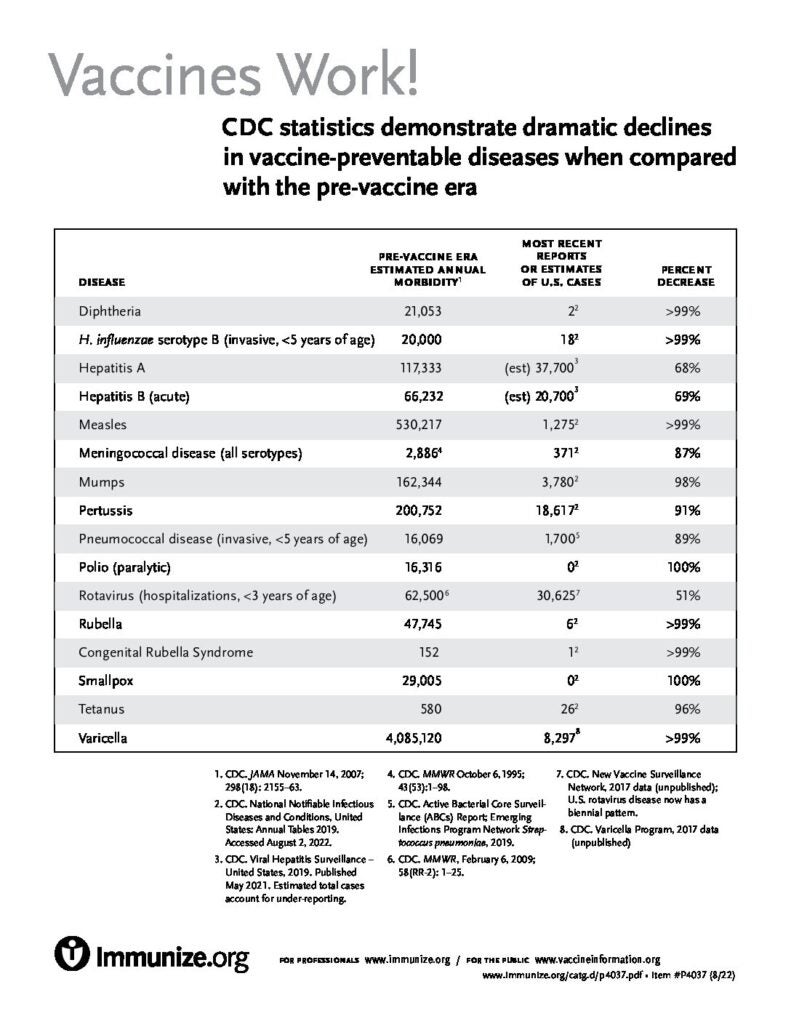
Vaccines Work!
CDC statistics demonstrate dramatic declines in vaccine-preventable diseases when compared with the pre-vaccine era
More From Immunize.org
Printable resources and links to partner organizations to help you address hesitancy related to vaccination-related anxiety.
Printable resources designed to help healthcare professionals in all aspects of immunization practice.
Immunize.org experts answer challenging questions about vaccines.
Information sheets produced by CDC that explain both the benefits and risks of a vaccine to vaccine recipients.
Real-life accounts of suffering and loss.
From our affiliated site VaccineInformation.org, information about the importance of vaccines and answers to many common questions.
CDC ∙ FDA ∙ WHO
CDC
Review how vaccines work, types of vaccines, and why some vaccines need more than one dose.
Connect with CDC’s resources about vaccine safety for healthcare providers, including storage and handling, recording adverse events, safety information by vaccine, and more.
Learn how various systems work together to ensure the safety of vaccines.
Read about the CDC’s Immunization Safety Office’s major programs to monitor vaccine safety, including the Vaccine Adverse Events Reporting System (VAERS), the Vaccine Safety Datalink (VSD), and the Clinical Immunization Safety Assessment Project (CISA).
Several fact sheets for healthcare professionals and patients that describe vaccine testing, monitoring, and how vaccines are added to the recommended U.S. immunization schedule.
A resource of vaccine safety research articles and reports, training information, and clinical resources, including some for patients.
CDC answers common questions parents ask about vaccine safety.
Before a new vaccine is ever given to people, extensive lab testing is done that can take several years. Once testing in people begins, it can take several more years before clinical studies are complete and the vaccine is licensed.
FDA
VAERS is a national vaccine safety surveillance program cosponsored by the FDA and CDC.
WHO
The GACVS meets twice a year to discuss and report on vaccine safety issues.
The Vaccine Safety Net is a global network of websites, established by the World Health Organization, that provides reliable information on vaccine safety.
Trusted Organizations
History of Vaccines (The College of Physicians of Philadelphia)
Types of side effects and how adverse events are monitored.
About the long, complex process of vaccine development and public and private involvement.
National Academy of Medicine
The National Academy of Medicine is an impartial group of the world’s leading experts that advises Congress on science issues.
A 2012 analysis of more than 1,000 research articles concludes that few health problems are caused by or clearly associated with vaccines.
A 2002 study from the National Academy of Medicine, an impartial group of the world’s leading experts that advises Congress on science issues.
Vaxopedia
A description of the process of monitoring vaccines to ensure safety.
The steps involved in manufacturing a vaccine.
Additional Resources
The institute provides independent assessments of vaccines and vaccine safety. From the Johns Hopkins Bloomberg School of Public Health.
A systematic review of the safety of U.S. recommended vaccines for children, adults, and pregnant women.
Vaccine Questions?
Email CDC at nipinfo@cdc.gov