Clinical Resources
Materials for Providers
Alphabetical by Title
Standing Orders for Administering Haemophilus influenzae Type B to Children & Teens
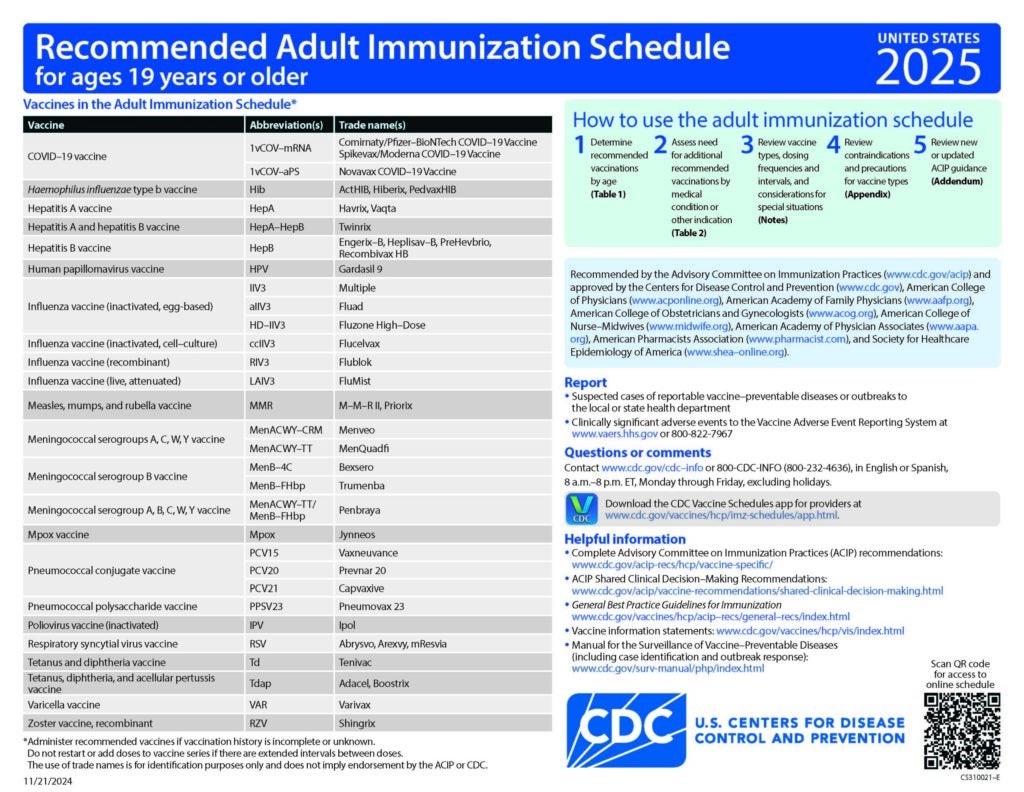
Eligible healthcare professionals may vaccinate children and teens who meet any of the criteria on this form. This template is consistent with the American Academy of Pediatrics 2026 Recommended Child and Adolescent Immunization Schedule.
CDC · FDA · State
ACIP Recommendations
Current Recommendations
Use of Haemophilus influenzae Type b–Containing Vaccines Among American Indian and Alaska Native Infants: Updated Recommendations of the Advisory Committee on Immunization Practices ― United States, 2024
MMWR September 12, 2024 / 73(36);799–802
Licensure of a Diphtheria and Tetanus Toxoids and Acellular Pertussis, Inactivated Poliovirus, Haemophilus influenzae Type b Conjugate, and Hepatitis B Vaccine, and Guidance for Use in Infants
MMWR, February 7, 2020, 69 (5);136-139
Prevention and Control of Haemophilus influenzae Type b Disease: Recommendations of the Advisory Committee on Immunization Practices (ACIP) Recommendations and Reports
MMWR, February 28, 2014; 63(RR01):1-14
Additional Federal Resources
CDC Recommended Schedules
FDA Package Inserts & EUAs
Hib: ActHIB Package Insert
Sanofi U.S.
Hib: Hiberix Package Insert
GSK
Hib: PedvaxHIB Package Insert
Merck & Co., Inc.
Combination Vaccine (DTaP-IPV/Hib): Pentacel Package Insert
Sanofi U.S.
Combination Vaccine (DTaP-IPV-Hib-HepB): Vaxelis Package Insert
MSP Vaccine Company