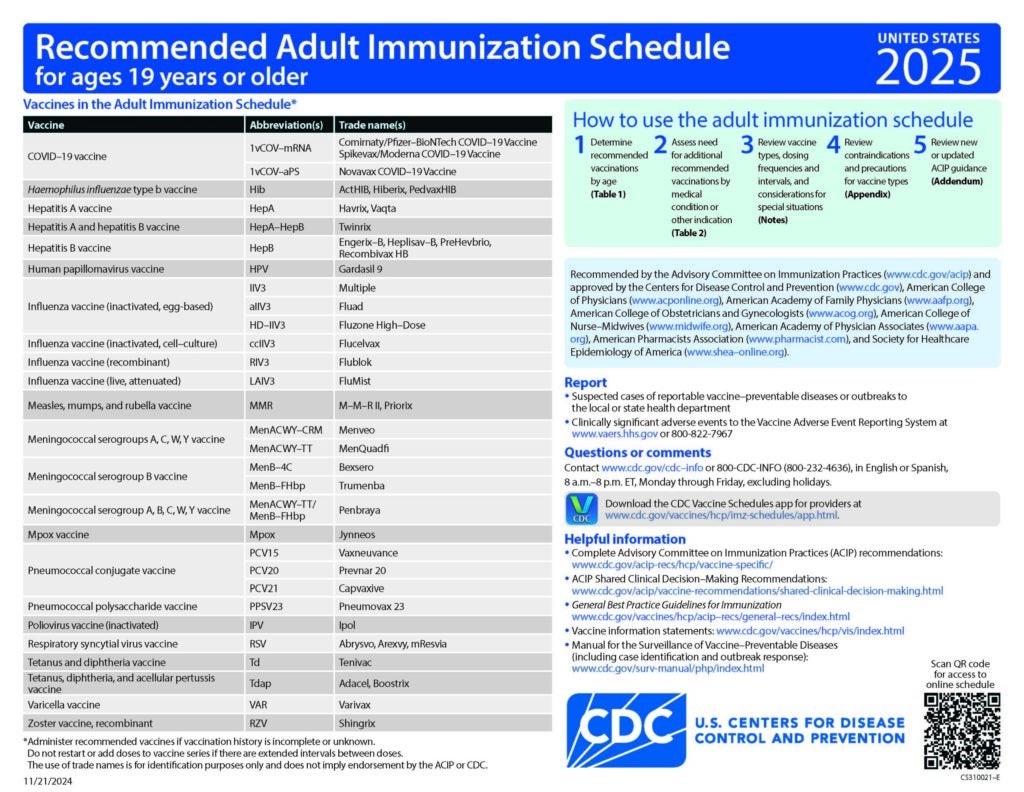
Recommended Immunization Schedules for Children, Adolescents, and Adults
Official immunization schedules, including timing of doses, for children, teens, and adults are available for download.
In our shop: Full-color laminated versions of each are created after CDC publishes the new annual schedules early in the year and are available for purchase while supplies last. Laminated schedules are durable and can be cleaned, helping them withstand a year of use in a busy clinic.
Shop Laminated Immunization Schedules →
Additional resources from CDC:
- Child and Adolescent Immunization Schedule by Age — Additional child and adolescent immunization schedule formats
- For You and Your Family — Parent-friendly schedules for birth to 6 years, and 7 to 18 years
- Schedule Changes & Guidance — Reference for updates to current schedules.
Healthcare professionals who recommend or administer vaccines can access all CDC recommended immunization schedules and footnotes using the CDC Vaccine Schedules app. Optimized for tablets and useful on smartphones, the app shows the child, adolescent, and adult vaccines recommended by the Advisory Committee on Immunization Practices (ACIP).