Clinical Resources
Materials for Providers
Alphabetical by Title
Checklist of Current Versions of U.S. COVID-19 Vaccination Guidance and Clinic Support Tools
Checklist of links to key COVID-19 vaccination resources, including the date they were last revised.
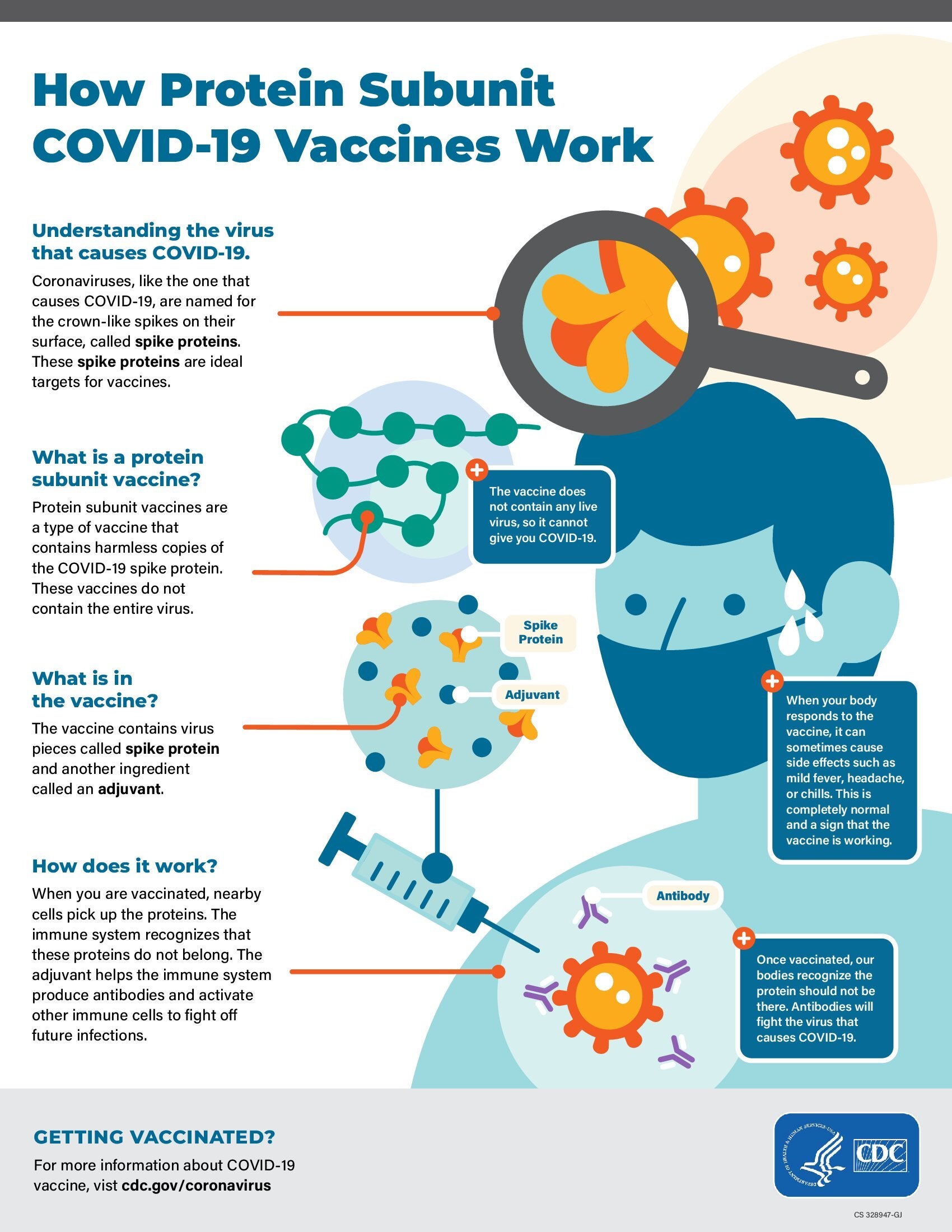
Standing Orders for Administering 2025-2026 Formula Adjuvanted Protein COVID-19 Vaccine to Individuals 12 Years and Older
Eligible healthcare professionals may administer Nuvaxovid (Sanofi-Novavax) COVID-19 vaccine to individuals 12 years of age and older, following clinical guidance from CDC (Interim Clinical Considerations for Use of COVID-19 Vaccines). A list of high-risk conditions supported by evidence is provided, along with a list of conditions that should trigger use of the dosing schedule for people with moderate or severe immunocompromise. CDC recommends COVID-19 vaccination of any person age 6 months or older following a shared clinical decision-making discussion. Links to current COVID-19 vaccine recommendations from AAP, AAFP, IDSA, and ACOG are also provided. These organizations recommend routine vaccination for designated people at increased risk of infection or severe disease and shared clinical decision-making for those at lower risk.
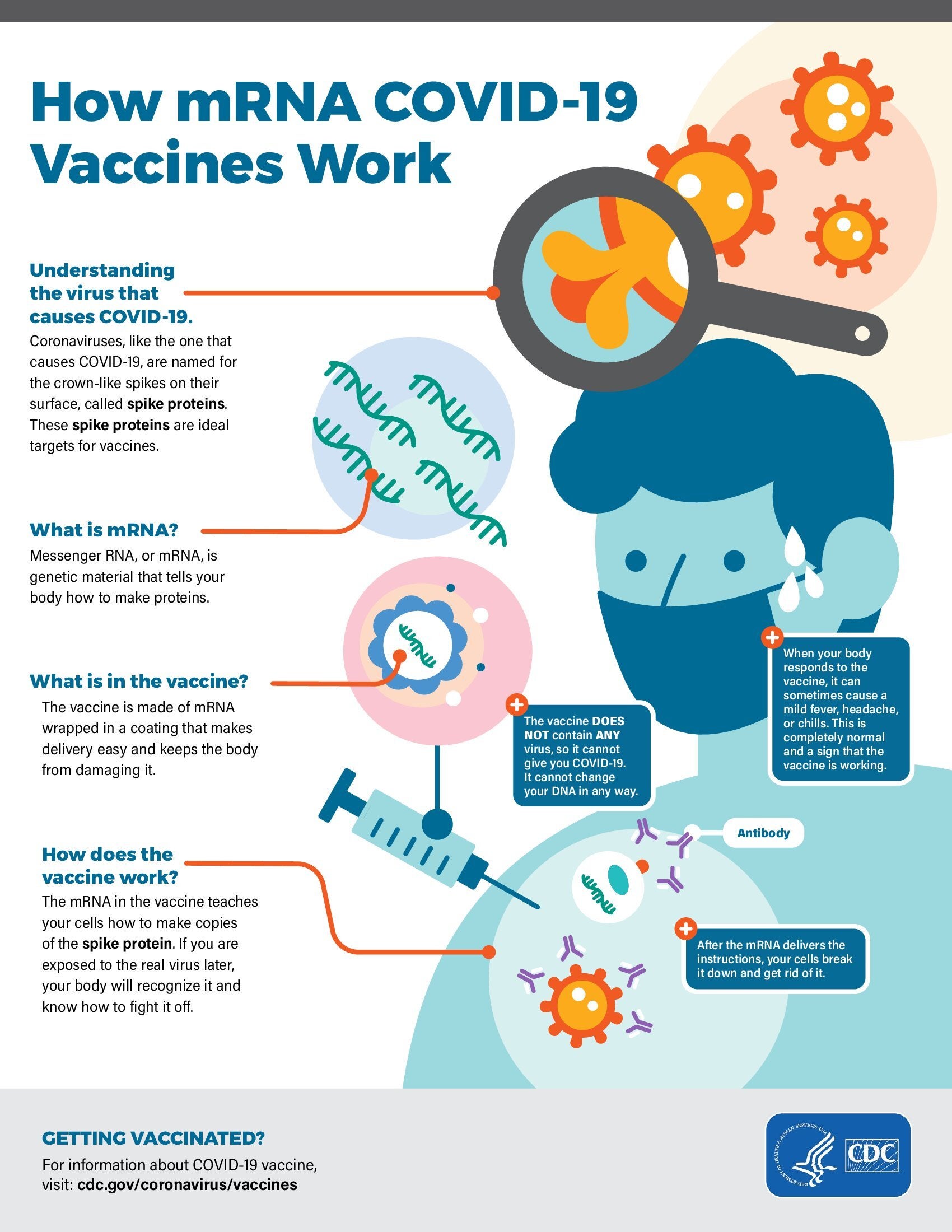
Standing Orders for Administering 2025-2026 Formula mRNA COVID-19 Vaccine to Children 6 Months through 11 Years
Eligible healthcare professionals may administer mRNA COVID-19 vaccines to children 6 months through 11 years, following clinical guidance from CDC (Interim Clinical Considerations for Use of COVID-19 Vaccines). A list of high-risk conditions supported by evidence is provided, along with a list of conditions that should trigger use of the dosing schedule for people with moderate or severe immunocompromise. CDC recommends COVID-19 vaccination of any person age 6 months or older following a shared clinical decision-making discussion. Links to current COVID-19 vaccine recommendations from AAP, AAFP, and IDSA are also provided. These organizations recommend routine vaccination for designated people at increased risk of infection or severe disease and shared clinical decision-making for those at lower risk.
Standing Orders for Administering 2025-2026 Formula mRNA COVID-19 Vaccine to Individuals 12 Years and Older
Eligible healthcare professionals may administer mRNA COVID-19 vaccines to individuals 12 years of age and older, following clinical guidance from CDC (Interim Clinical Considerations for Use of COVID-19 Vaccines). A list of high-risk conditions supported by evidence is provided, along with a list of conditions that should trigger use of the dosing schedule for people with moderate or severe immunocompromise. CDC recommends COVID-19 vaccination of any person age 6 months or older following a shared clinical decision-making discussion. Links to current COVID-19 vaccine recommendations from AAP, AAFP, IDSA, and ACOG are also provided. These organizations recommend routine vaccination for designated people at increased risk of infection or severe disease and shared clinical decision-making for those at lower risk.
Ask the Experts
CDC · FDA · State
ACIP Recommendations
Current Recommendations
Additional Federal Resources
- All current and archived ACIP COVID-19 recommendations
- ACIP COVID-19 recommendations at CDC
- Interim Clinical Considerations for Use of COVID-19 Vaccines in the United States (CDC)
- Clinical Considerations: Myocarditis and Pericarditis after Receipt of mRNA COVID-19 Vaccines Among Adolescents and Young Adults (CDC)
- Vaccine Storage and Handling Toolkit (CDC)
- General Best Practices for Immunization
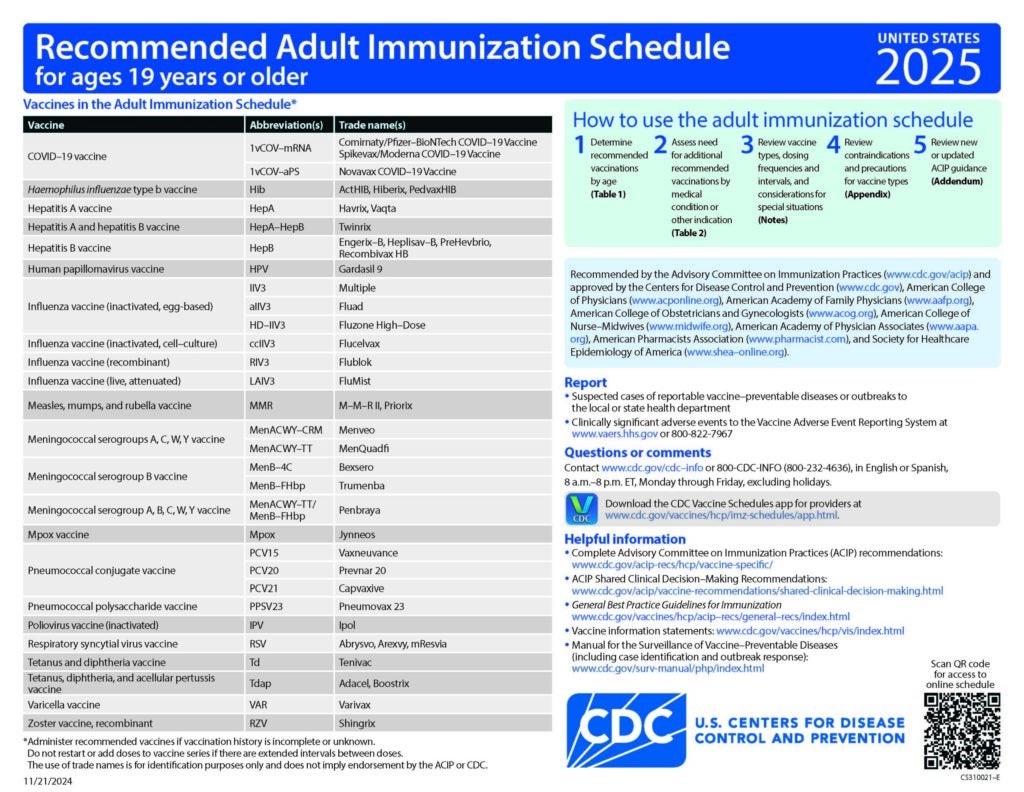
CDC Recommended Schedules
FDA Package Inserts & Vaccine Fact Sheets (EUA)
State Policies
Healthcare Professional Organizations
Vaccine Recommendations
Travel
All travelers should be up to date on routine vaccines. Depending on the destination, itinerary, and duration of travel, additional vaccines may be recommended.
CDC Resources
Travelers’ health information for healthcare providers