- American Academy of Pediatrics releases its 2026 Recommended Child and Adolescent Immunization Schedule, endorsed by 12 other medical, nursing, and pharmacy organizations
- Reminder: Please take Immunize.org’s biennial 5-minute feedback survey
- "Measles Outbreak Associated with an Infectious Traveler—Colorado, May–June 2025" published in MMWR
- Measles 2026: 588 confirmed cases in 17 states since January 1; South Carolina outbreak growing
- Influenza and RSV are increasing nationwide; low immunization rates leave infants and adults vulnerable
- "Public health is science with a moral compass": remembering William H. Foege, MD, CDC director and leader in smallpox eradication
- "Vaccination is love in action": remembering Alan R. Hinman, MD, MPH, and a life dedicated to public health and vaccinology
- “When Is the Optimal Timing for RSV Preventive Antibody Administration?” Watch the new 2-minute answer, part of the Ask the Experts Video Series on YouTube.
- Vaccines in the news
On January 26, the American Academy of Pediatrics (AAP) released its 2026 Recommended Child and Adolescent Immunization Schedule. The new AAP guidance continues to recommend routine vaccination against 18 vaccine-preventable diseases, largely unchanged from the original 2025 CDC schedule published in November 2024. In May 2025, AAP stopped endorsing the modified 2025 CDC schedule after CDC made changes to the COVID-19 vaccination recommendation. The CDC’s 2026 schedule narrowed the number of vaccines recommended routinely for children, moving COVID-19, HepA, HepB, influenza, MenACWY, and rotavirus vaccines into shared clinical decision-making or risk-based categories. Three other differences are notable:
- MMR and Varicella: AAP continues to support the option to use combination MMRV vaccine (ProQuad, Merck) for a child’s first dose of measles, mumps, rubella, and varicella antigen. In June 2025, CDC began to recommend only giving MMR vaccine plus monovalent varicella vaccine at separate injection sites to children younger than age 5 years.
- HPV: AAP continues the long-standing recommendation for two doses of HPV vaccine routinely given at age 9–12 years, while the 2026 CDC schedule recommends one dose at 11–12 years routinely.
- COVID-19: In August 2025, AAP updated its COVID-19 guidance, recommending that all young children age 6–23 months be vaccinated routinely, along with older children in certain risk groups. The 2026 CDC schedule groups COVID-19 vaccine under shared clinical decision-making for all children age 6 months or older.
All immunizations recommended on the AAP schedule are also listed on the CDC schedule, although the classification of the recommendations may differ. HHS has publicly stated that all immunizations recommended by the CDC as of December 31, 2025, before CDC issued the modified 2026 schedule, will continue to be covered by Affordable Care Act insurance plans and federal insurance programs, including Medicaid, the Children’s Health Insurance Program, and the VFC program. Families will not have to purchase them out of pocket.
The 2026 AAP immunization schedule was endorsed by 12 medical, nursing, and pharmacy organizations representing more than 1 million healthcare professionals, including:
- American Academy of Family Physicians (AAFP)
- American College of Nurse Midwives (ACNM)
- American College of Obstetricians and Gynecologists (ACOG)
- American Medical Association (AMA)
- American Pharmacists Association (APhA)
- Council of Medical Specialty Societies (CMSS)
- Infectious Diseases Society of America (IDSA)
- National Association of Pediatric Nurse Practitioners (NAPNAP)
- National Medical Association (NMA)
- Pediatric Infectious Diseases Society (PIDS)
- Pediatric Pharmacy Association (PPA)
- Society for Adolescent Health and Medicine (SAHM)
- AAP: 2026 Recommended Child and Adolescent Immunization Schedule (PDF)
- AAP news release: American Academy of Pediatrics Issues Recommended Childhood and Adolescent Immunization Schedule for 2026 (1/26/26)
- AAP: 12 Medical Groups Representing 1 Million Health Care Professionals Endorse AAP Immunization Schedule (1/26/26)
- AAP: AAP’s 2026 Immunization Schedule Keeps Routine Recommendations Intact After Overhaul of Federal Schedule (1/26/26)
- Immunize.org: Official Guidance: Healthcare Professional Organizations main page
Once every 2 years, Immunize.org asks you to respond to a brief online survey on the aspects of our work that matter to you. This may include our communications, clinical resources, translations, coalition support, or websites.
Your anonymous feedback is vital to our strategic planning and program evaluation process. Your responses also help us show the value of the work we do with the funding we receive.
SurveyMonkey will skip sections you mark as not relevant to you. Answering all sections may take as little as 5 minutes. The survey will be open until February 11. Thank you for sharing your thoughts with us.
Click here to complete the 2026 Immunize.org evaluation survey now!
CDC published Measles Outbreak Associated with an Infectious Traveler—Colorado, May–June 2025 on January 29 in MMWR. The report highlights the spread of measles from an infected traveler (index case) to people who contracted measles from the index case (secondary cases) and to people who contracted measles from a secondary case (tertiary cases). A portion of the summary appears below.
Measles is a highly contagious vaccine-preventable disease. Two measles, mumps, and rubella (MMR) vaccine doses are 97% effective in preventing measles. . . .
During May 25–June 7, 2025, nine secondary measles cases and one tertiary case occurred, including four patients who were hospitalized, among Colorado residents exposed during an international flight or in an airport to an air traveler with infectious measles who had acquired the disease in the United States. Seven additional cases were reported by other jurisdictions. In two vaccinated patients, the virus was detected only in urine and not in nasopharyngeal specimens. . . .
All eligible persons should receive 2 MMR vaccine doses. Travelers should ensure that their MMR vaccinations are current. Collecting urine specimens might enhance case finding, especially in vaccinated persons.
Access the MMWR article in HTML or PDF.
Related Link
- CDC: MMWR main page providing access to the MMWR family of publications
CDC only requires reporting of laboratory-confirmed measles cases. Cases without laboratory testing for confirmation are not included in these numbers. Actual numbers of cases are, therefore, higher than confirmed case counts.
A map of 2025–26 measles cases in the United States, as of January 30, from the Johns Hopkins International Vaccine Access Center, appears below. Their U.S. Measles Tracker website includes state and county-level data.
Immunize.org offers measles-related resources for the public on several of our affiliated websites:
- VaccineInformation.org: Measles web page
- LetsGetRealAboutVaccines.org: Measles web page
- Immunize.org: Vaccines A–Z: Measles main page
- AAP: Fact Checked: The Measles Vaccine Is Safe and Effective web page
- CDC: Measles Cases and Outbreaks main page
- CDC: Be Ready for Measles toolkit
- Health Canada: Measles and Rubella Weekly Monitoring Report
- Johns Hopkins Bloomberg School of Public Health Center for Outbreak Response Innovation: Measles Outbreak Response main page
- Immunize.org: Vaccines A–Z: Measles main page
Influenza and RSV are increasing nationwide; low immunization rates leave infants and adults vulnerable
Nationwide respiratory virus activity reported by CDC is highlighted below.
- Influenza (data through January 24):
- Seasonal influenza activity increased this week after declining for 3 weeks.
- High or very high influenza-like illness (ILI) was reported in 29 jurisdictions.
- The influenza season is currently classified as moderate overall, but severe for young children.
- The deaths of 8 more children were reported during week 3 (ending January 24), for a total of 52 reported child deaths associated with influenza so far this season.
- RSV:
- The Epidemic Trends map shows RSV activity growing or likely growing in 22 states, including most of the Midwest and western regions, as well as Maine, New Hampshire, and Vermont.
- Respiratory Illness Data Channel shows the incidence of emergency department (ED) visits for RSV is elevated among infants younger than age 1 year.
- Use of RSV preventive antibodies in susceptible infants younger than age 8 months and high-risk children age 8 through 19 months will protect them now. Many eligible children remain unprotected as RSV rises.
- COVID-19: Activity varies by state. Adults age 65 or older represented 71% of those hospitalized for COVID-19 during week 3.
Level of Respiratory Illness Activity
Because influenza is leading the respiratory illness wave, the ILI map is shown below:

Emergency Department (ED) Visits for Viral Respiratory Illness
The illustration below shows the proportion of ED visits (ranging from 0 to 10 percent) associated with COVID-19, influenza, and RSV. The horizontal axis shows trends from October 2024 into January 2026 for the three diseases.

It’s not too late to vaccinate! Vaccination against COVID-19, influenza, and RSV reduces the risk of severe illness. Administration of RSV preventive antibodies for infants younger than 8 months who are unprotected is crucial now to provide them immediate protection as RSV activity rises in many communities.
Other CDC Respiratory Illness Resources- CDC's Respiratory Illness Data Channel shows state and county level data on respiratory viral activity, associated ED visits, and presence in wastewater
- CDC's Weekly Flu Vaccination Dashboard shows that as of January 10, 133.5 million doses of influenza vaccine were distributed in the U.S., compared to 145.5 million doses last season
- The National Immunization Survey–Fall Respiratory Virus Module show that influenza vaccination coverage varied by age, health insurance status, poverty status, race and ethnicity, urbanicity, sex, and jurisdiction
Related Links
- CDC: Weekly National Flu Vaccination Dashboard main page
- CDC: FluView main page
- CDC: RESP-NET main page
- CDC: FluVaxView main page
- 65+ Flu Defense website
"Public health is science with a moral compass": remembering William H. Foege, MD, CDC director and leader in smallpox eradication
William “Bill” H. Foege, MD, MPH, whose work in public health transformed the global response to infectious diseases, died on January 24, at age 89. Best known for his role in eradicating smallpox, which killed 300 million people in the 20th century alone, Dr. Foege helped lead one of the greatest scientific and public health achievements in history. While working as a CDC epidemiologist in West Africa during the 1960s, he pioneered the strategy of “ring vaccination,” vaccinating everyone within a ring around the infected person. This approach became the foundation of the WHO’s global smallpox eradication campaign in 1966, leading to the eradication of smallpox declared in May 1980.

Dr. Foege served as CDC director from 1977 to 1983, strengthening immunization programs and advancing the agency’s role in global health. Throughout his career, he championed vaccines as one of the most powerful tools in medicine, helped expand childhood immunization efforts worldwide, and shaped outbreak response strategies still used today. A recipient of the Presidential Medal of Freedom, Dr. Foege also helped establish the Task Force for Global Health and served in leadership roles at the Carter Center and the Bill and Melinda Gates Foundation. His scientific insight, vision, and belief in the lifesaving power of vaccines will continue to protect generations to come.
For more about Dr. Foege's remarkable life, please see his obituary in the New York Times: William H. Foege, Key Figure in the Eradication of Smallpox, Dies at 89.
"Vaccination is love in action": remembering Alan R. Hinman, MD, MPH, and a life dedicated to public health and vaccinology
Alan R. Hinman, MD, MPH, spent more than six decades strengthening immunization programs and advancing the prevention of vaccine-preventable diseases in the United States and globally. After training with CDC’s Epidemic Intelligence Service, his senior leadership roles at the CDC included serving as director of the Immunization Division (1977–1988). Dr. Hinman rose to the rank of assistant surgeon general. His work helped shape national immunization policy, expand school vaccination requirements, establish and improve the essential functions of immunization information systems (i.e., immunization registries), and support progress toward measles elimination and polio eradication.
Following government service, Dr. Hinman worked with The Task Force for Global Health, served on the board of Gavi, The Vaccine Alliance, and chaired a WHO advisory committee. He authored or coauthored more than 400 publications, and mentored future public health leaders as an adjunct professor of global health and epidemiology at the Rollins School of Public Health at Emory University. He was also a founder of Voices for Vaccines, a program of The Task Force for Global Health and our partner in sharing accurate vaccination information with families.

Dr. Hinman passed away at age 88 on January 26. He was a great friend to many of us across the public health and immunization community. His family noted in his obituary, “Alan would be the first to remind us that public health is, at heart, an act of love: for neighbors you know, and neighbors you will never meet. If you're looking for a way to honor him, consider doing something wonderfully Alan-ish: tell the truth clearly, show up consistently, share a good meal with smart people, and yes keep your vaccinations up to date. He spent a lifetime making sure the world had a fighting chance.”
He will be missed.
Read Dr. Hinman's obituary.
“When Is the Optimal Timing for RSV Preventive Antibody Administration?” Watch the new 2-minute answer, part of the Ask the Experts Video Series on YouTube.
This week, our featured episode from the Ask the Experts Video Series is titled When Is the Optimal Timing for RSV Preventive Antibody Administration? The video explains that to protect infants during their first RSV season, the optimal timing for nirsevimab (Beyfortus, Sanofi) or clesrovimab (Enflonsia, Merck) dosing is shortly before the RSV season begins. However, because of their immediate effect, these products should be administered to eligible infants and children at any time during the season, even when optimal timing is missed.
RSV preventive antibody products are recommended during the months of October through March in most areas in the United States, unless otherwise advised by state or territorial public health authorities. Adjustment of timing due to RSV activity outside this typical timeframe is most likely to be necessary in tropical or subtropical climates and in Alaska.
Only nirsevimab (Beyfortus) is recommended for high-risk children in their second RSV season.
The 2-minute video is available on our YouTube channel, along with our full collection of quick video answers to popular Ask the Experts questions.

Like, follow, and share Immunize.org’s social media accounts and encourage colleagues and others interested in vaccination to do likewise.
- Facebook at ImmunizeOrg
- Instagram at ImmunizeOrg
- LinkedIn at ImmunizeOrg
- YouTube at ImmunizeOrg
These recent articles convey the potential risks of vaccine-preventable diseases and the importance of vaccination.
- Washington Post: What South Carolina’s Soaring Measles Outbreak Means for the Rest of the U.S. (1/31/26)
- Your Local Epidemiologist: Shared Decision-Making, Informed Consent, and the Rhetoric of False Empowerment (1/28/26)
- AMA: Pediatric Vaccines: Questions Parents Will Ask—and How to Answer (1/28/26)
- Medpage Today, Opinion: Flu Season Is a Stress Test. Our Healthcare System Keeps Failing. (1/27/26)
![]()
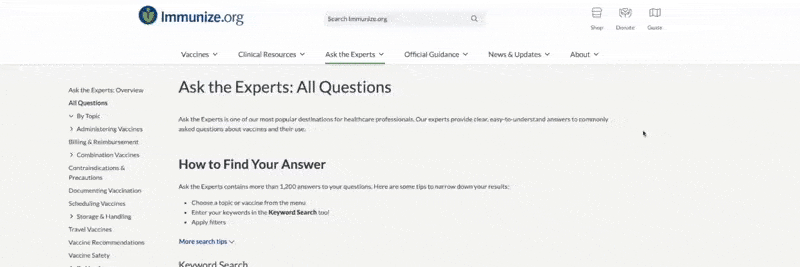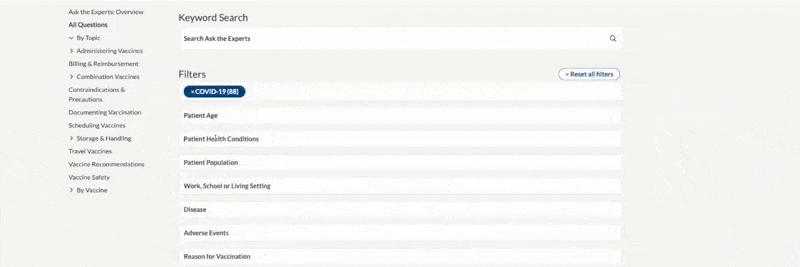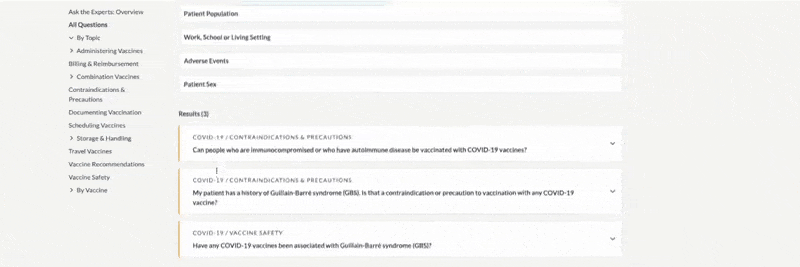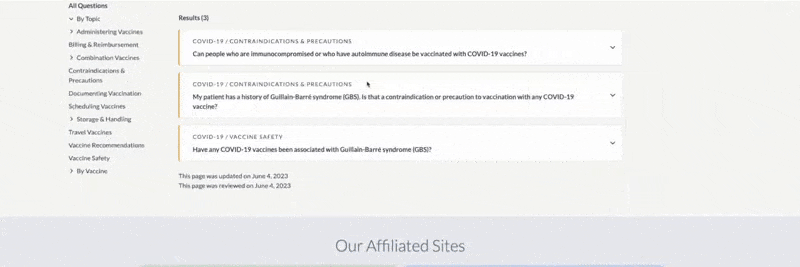
Immunize.org’s Clinical Resources A–Z section includes 174 printable resources (plus more than 120 translated documents) grouped under one of 20 topics or by audience:
- All clinical resources
- Patient handouts
- Resources for staff and providers
To view content, select Clinical Resources from atop any web page. On the left side, click Clinical Resources A–Z. From there, you can choose the audience group or one of 20 listed topics, such as Standing Orders Templates.

Within any search option, narrow the results by:
- Entering keywords
- Applying a filter
- Picking a topic from the left navigation menu
The example below shows that the topic Standing Orders Templates was clicked, along with the filter “influenza,” which resulted in two documents.

Results are listed alphabetically by title and can also be sorted by item number or by version date. For a more compact display, you can hide document previews by toggling the Hide/Show Images button. View the document by clicking the title or the thumbnail image. The right side of the card lists the document’s version date and available languages. You can also filter for languages or choose Translations from the left menu.
On a mobile device, navigate to the main menu by clicking the three horizontal lines in the top right corner. On the screens that pop up, click on Clinical Resources, then on Clinical Resources A–Z, and then scroll down the menu to choose your desired selection to view options.

Where can I find more information about Clinical Resources?
Learn more by watching a short orientation video (customized for desktop or mobile view). For a deeper dive, we offer a 37-minute webinar to explore content and demonstrate navigation of Clinical Resources.
- Orientation Video Series: Introducing Clinical Resources (3:37 minutes)
- Orientation Video Series: Introducing Clinical Resources (Mobile View) (3:29 minutes)
- Website Office Hours: Clinical Resources (37:48 minutes)
Immunize.org made a minor change to its Screening Checklist for Contraindications to HPV, MenACWY, MenB, and Tdap Vaccines for Teens to specify that ACOG recommends injectable influenza vaccine, COVID-19 vaccine, Tdap, and RSV vaccines during pregnancy. No changes to the contraindications or the questionnaire for patients were made.

Related Links
- Immunize.org: Clinical Resources: Screening Checklists main page
- Immunize.org: Clinical Resources A–Z main page, where you can filter by topic, vaccine, language, or other criteria
IZ Express regularly provides readers with information about Immunize.org’s new and updated educational materials for healthcare professionals and patient handouts. All Immunize.org materials are free to distribute.
In case you missed them during recent weeks, the following updated materials were posted for clinicians:
- Don’t be Guilty of These Preventable Errors in Vaccine Administration!
- Standing Orders for Administering Meningococcal B (MenB) Vaccine to Adolescents and Adults
Information to share with patients:
- What If You Don’t Vaccinate Your Child?
- Top Ten Reasons to Protect Your Child by Vaccinating
- Measles: Questions and Answers
- Mumps: Questions and Answers
- Rubella: Questions and Answers
- Varicella (Chickenpox): Questions and Answers
- Meningococcal: Questions and Answers
Website sections:
- New! Healthcare Professional Organization Schedules
- New! Healthcare Professional Organization Vaccine Recommendations
- Ask the Experts: Vaccine Recommendations
- Ask the Experts: COVID-19
- Vaccines A–Z: COVID-19
Website Office Hours webinars and training videos:
- Website Office Hours: Image Library, Webinars, Videos, and Social Media (25:31)
- Website Training Videos: Image Library, Webinars, Videos, and Social Media (18:00)
- Website Office Hours: Vaccines A–Z (24:16)
- Website Training Videos: Vaccines A–Z (12:21)
The CHOP's Vaccine Education Center released the second season of its VEC Vaccine Notes podcast series, which relays vaccine information in both video and podcast formats for people on the go. Each episode is based on one of VEC’s Vaccines and Diseases web pages. Each describes the diseases vaccines prevent, the vaccines available, answers to common questions, and vaccination risks and benefits.
Episodes so far this season covered HPV, pneumococcus, anthrax, and rotavirus. In the coming weeks, VEC Vaccine Notes will cover chikungunya, rabies, mpox, and more.
VEC Vaccine Notes is available on YouTube and can be listened to via podcast at: Podbean, Apple Podcasts, Spotify, iHeartRadio, and PodChaser.

View the VEC Vaccine Notes web page for more.
Confident healthcare provider recommendations for influenza vaccine are powerfully persuasive. As the nation faces a challenging influenza season, Immunize.org, in collaboration with CSL Seqirus, updated the 65+ Flu Defense website to help you maximize patient protection.

This helpful site includes information, tools, and tips for communicating with adults age 65 and older about the burden and severity of influenza. Resources include:
- Influenza in Adults 65+: The Facts
- Influenza Vaccination: Questions Patients Aged 65 and Older Frequently Ask Their HCP
- The Importance of Preventing Influenza and COVID-19
A clinician recommendation is the most important reason why a person will get vaccinated. Check out the updated 65+ Flu Defense website to assist your ongoing efforts in protecting this vulnerable population.
On January 26, WHO announced that six European countries (United Kingdom, Spain, Austria, Armenia, Azerbaijan, and Uzbekistan) are no longer considered measles-free. This step is generally taken after a country experiences at least one year of sustained measles transmission. A spike in measles cases in 2024 pushed these countries into the same category as France, Romania, and others, where ongoing transmission has become more common. Although WHO finalized the status changes for the affected European countries in September 2025, the announcement was delayed until all governments formally signed off on the status change.
Measles is currently circulating in a quarter of the WHO’s 53-country European region, with most reported cases occurring in people who are unvaccinated or under-immunized. High vaccination coverage is the only effective way to prevent measles outbreaks. At least 95% coverage is needed, along with strong epidemiologic surveillance to rapidly detect and respond to cases.
- Vaccines Today: Measles 'Reestablished' in Six European Countries (1/26/26)
- Vaccines Today: Measles in Europe: How to Get Back on Track (1/26/26)
- Reuters: European Countries Including UK Lose Measles Elimination Status (1/26/26)
- Immunize.org: Vaccines A–Z: Measles main page
To learn simple tips and tricks for using our websites efficiently, please register for our next set of Website Office Hours on Wednesday, February 11, at 4:00 p.m. (ET) or Thursday, February 12, at 12:00 p.m. (ET). The same content will be covered in both sessions.
We will open each 30-minute session with a short, live demonstration on navigating our new Official Guidance: Healthcare Professional Organization Schedules website section. You can submit questions when you register or live on Zoom during the session.

Register today for Immunize.org Website Office Hours (content is the same for both):
The archive of previous Website Office Hours content is posted at Immunize.org’s "Webinars & Videos" page.
Mark your calendar for future Immunize.org Website Office Hours.
For more upcoming events, visit our Calendar of Events.
About IZ Express
IZ Express is supported in part by Grant No. NH23IP922654 from CDC’s National Center for Immunization and Respiratory Diseases. Its contents are solely the responsibility of Immunize.org and do not necessarily represent the official views of CDC.
IZ Express Disclaimer
ISSN 2771-8085
Editorial Information
-
Editor-in-ChiefKelly L. Moore, MD, MPH
-
Managing EditorJohn D. Grabenstein, RPh, PhD
-
Associate EditorSharon G. Humiston, MD, MPH
-
Writer/Publication CoordinatorTaryn Chapman, MS
Courtnay Londo, MA -
Style and Copy EditorMarian Deegan, JD
-
Web Edition ManagersArkady Shakhnovich
Jermaine Royes -
Technical ReviewerKayla Ohlde