Clinical Resources
Materials for Providers
Alphabetical by Title
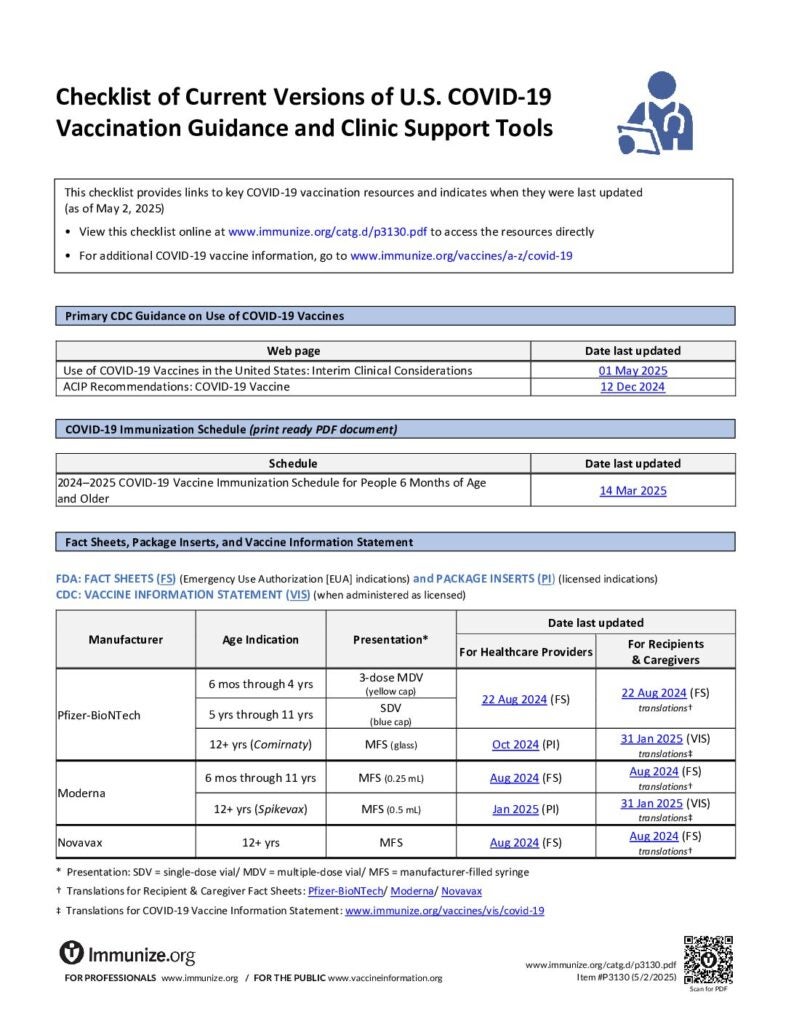
Checklist of Current Versions of U.S. COVID-19 Vaccination Guidance and Clinic Support Tools
Checklist of links to key COVID-19 vaccination resources, including the date they were last revised. Updated at least monthly.
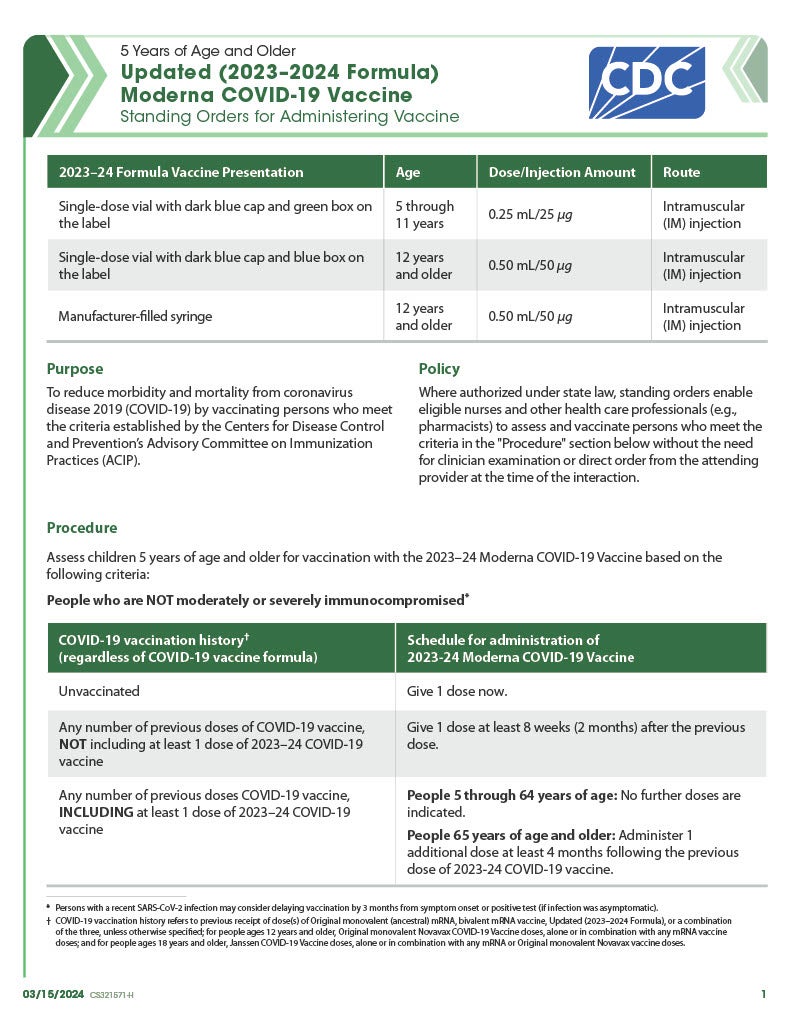
COVID-19 Moderna Updated (2023–2024 Formula) Vaccine – 5 Years of Age and Older (Age 5 Through 11 Yrs – Dark Blue Cap/Green Label; Age 12+ Yrs – Dark Blue Cap/Blue Label and Manufacturer-filled Syringe)
CDC’s form: “Updated (2023–2024 Formula) Moderna COVID-19 Vaccine: Standing Orders for Administering Vaccine – 5 Years of Age and Older”
COVID-19 Moderna Updated (2023–2024 Formula) Vaccine – 6 Months Through 4 Years of Age (Dark Blue Cap/Green Label)
CDC’s form: “Updated (2023–2024 Formula) Moderna COVID-19 Vaccine: Standing Orders for Administering Vaccine – 6 Months Through 4 Years of Age”
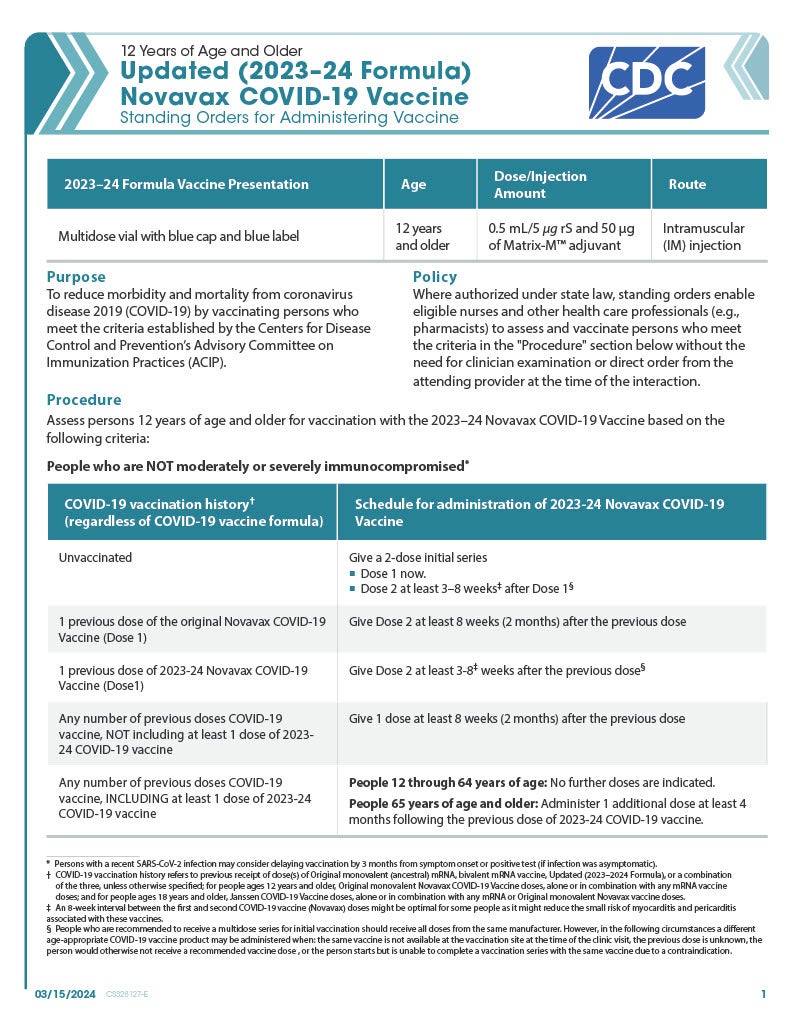
COVID-19 Novavax Updated (2023-24 Formula) 12 Years of Age and Older (Multidose Vial with Blue Cap/Blue Label)
CDC’s form: “Updated (2023-24 Formula) Novavax COVID-19 Vaccine Standing Orders for Administering Vaccine to Persons 12 Years of Age and Older”
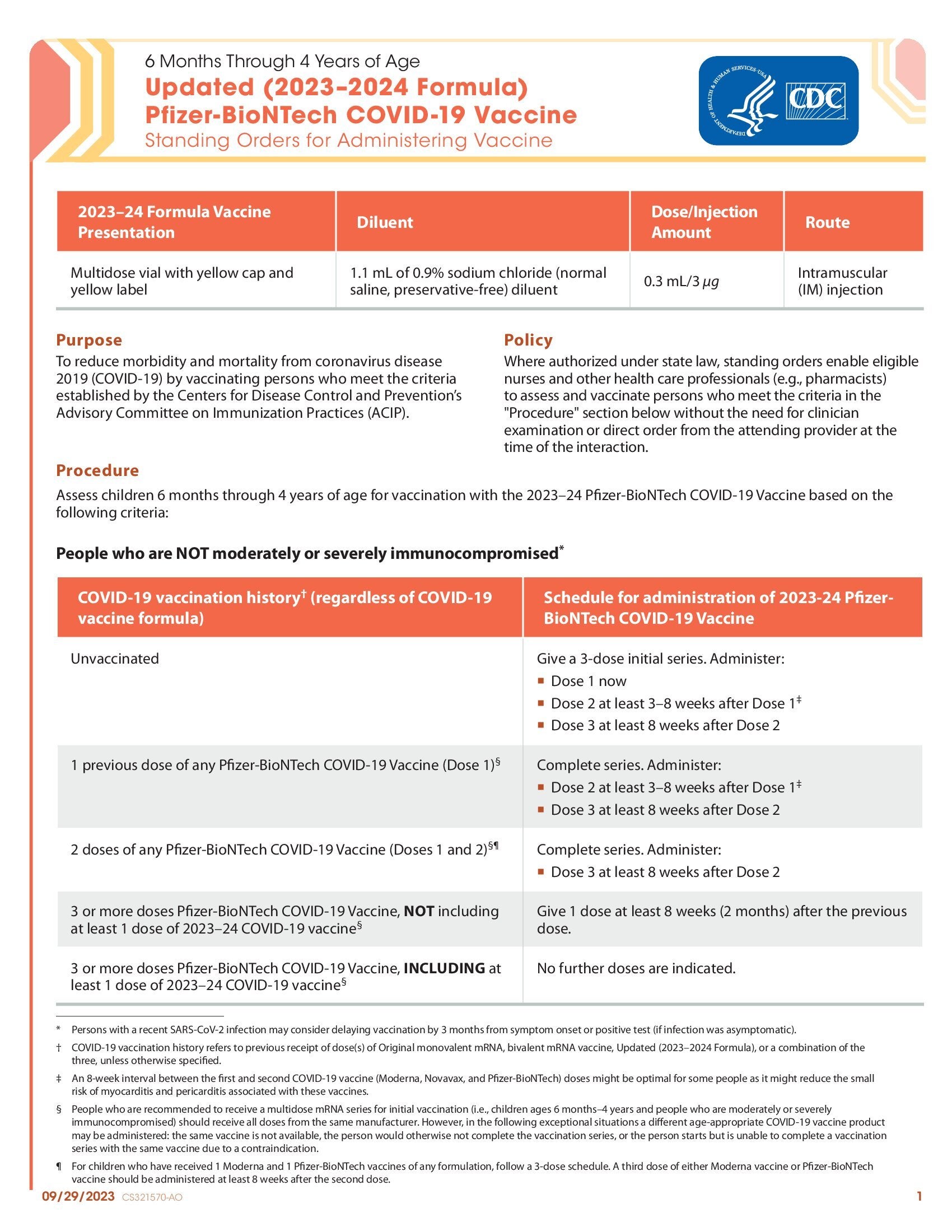
CDC’s form: “Updated (2023–2024 Formula) Pfizer-BioNTech COVID-19 Vaccine: Standing Orders for Administering Vaccine – 6 Months Through 4 Years of Age”
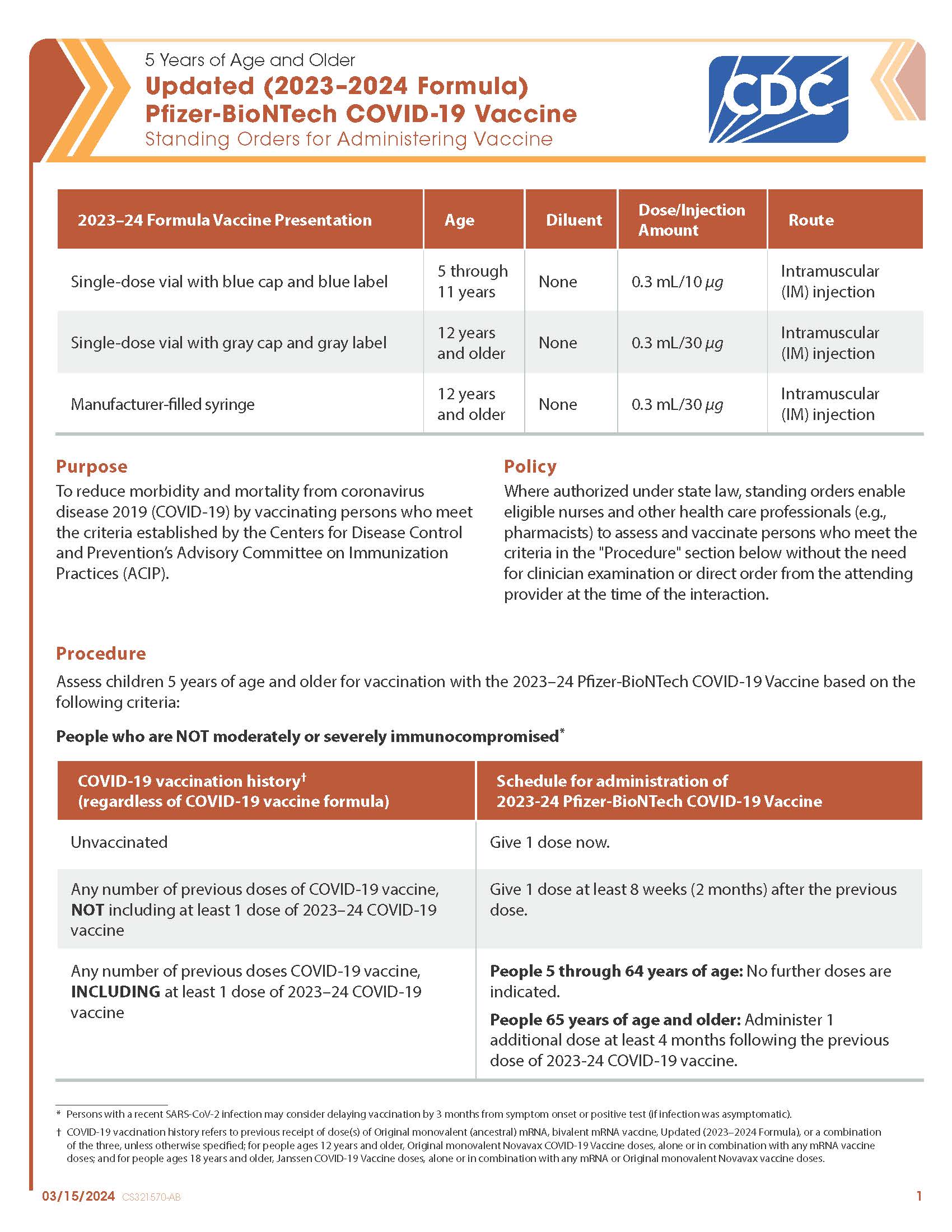
COVID-19 Pfizer-BioNTech Updated (2023–2024 Formula) Vaccine — 5 Years of Age and Older (Blue Cap/Blue Label, Gray Cap/Gray Label, Manufacturer-filled Syringe)
CDC’s form: “Updated (2023–2024 Formula) Pfizer-BioNTech COVID-19 Vaccine: Standing Orders for Administering Vaccine – 5 Years of Age and Older”
Ask the Experts
CDC · FDA · State
ACIP Recommendations
Current Recommendations
Additional Federal Resources
- All current and archived ACIP COVID-19 recommendations
- ACIP COVID-19 recommendations at CDC
- Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Approved or Authorized in the United States (CDC)
- COVID-19 Vaccination Clinical & Professional Resources (CDC) [main page]
- U.S. COVID-19 Vaccine Product Information (CDC)
- Clinical Care Considerations for COVID-19 Vaccination (CDC)
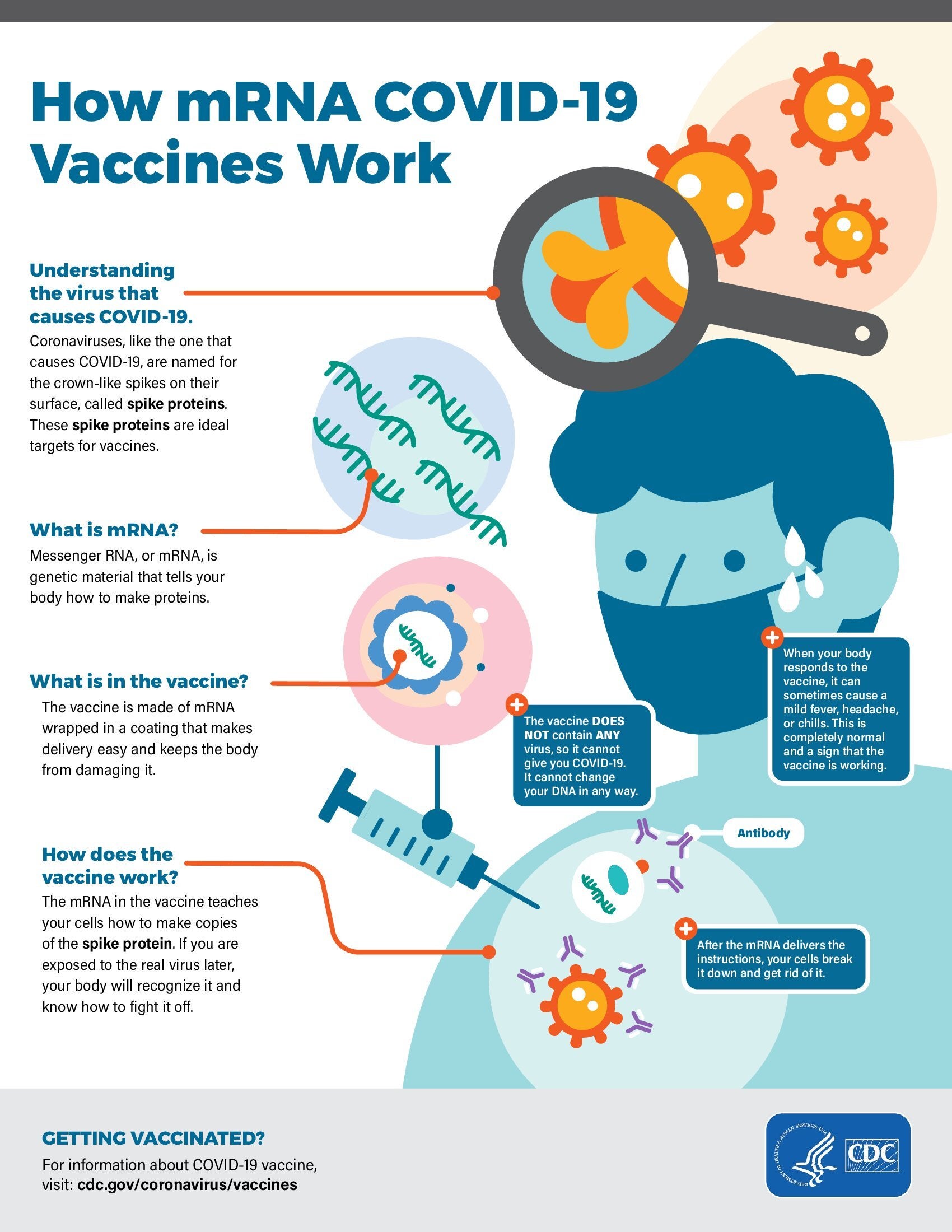
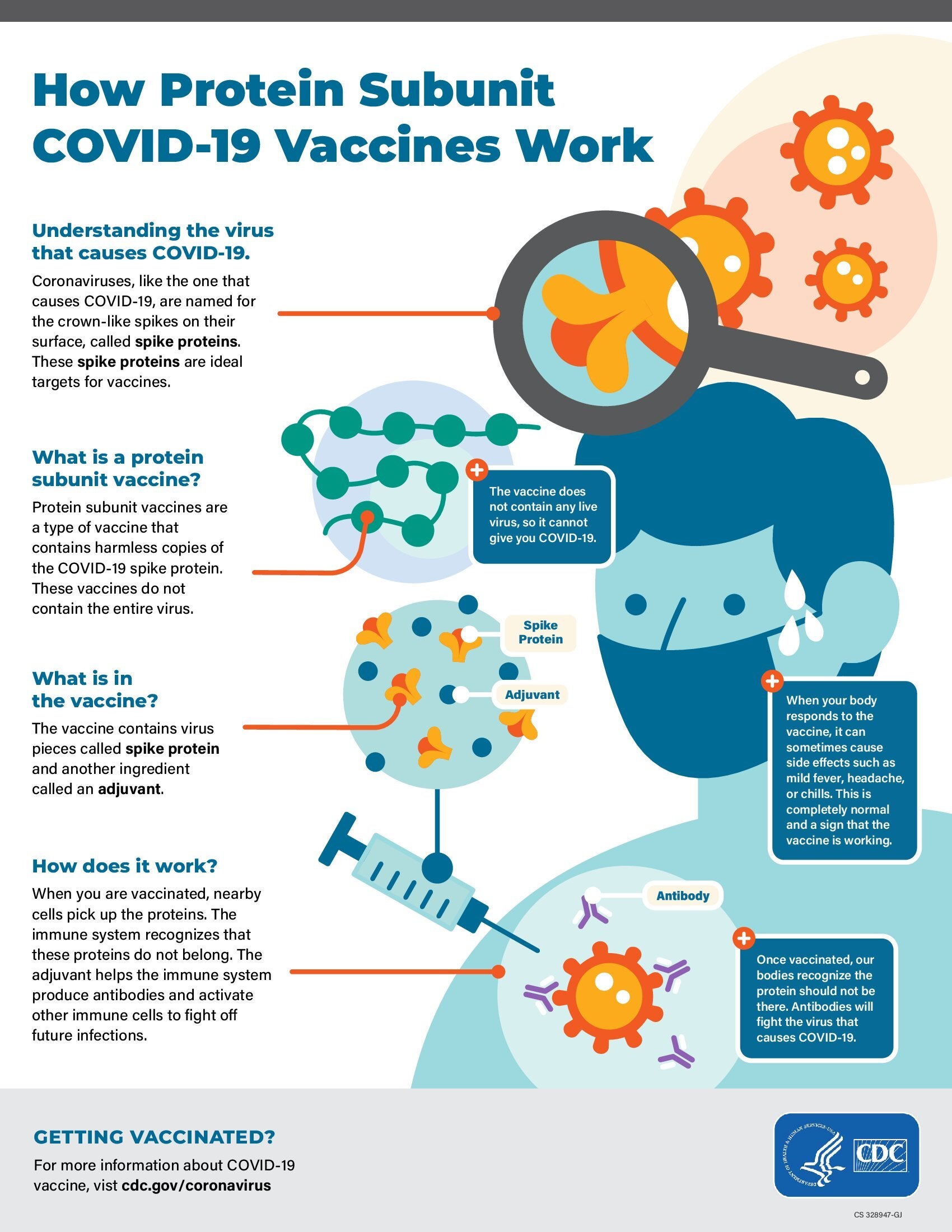
- Clinical Considerations: Myocarditis and Pericarditis after Receipt of mRNA COVID-19 Vaccines Among Adolescents and Young Adults (CDC)
- Communication and Print Resources (CDC)
- Vaccinate with Confidence: Strategy to Reinforce Confidence in COVID-19 Vaccines (CDC)
- Ensuring COVID-19 Vaccine Safety in the US (CDC)
- Vaccine Storage and Handling Toolkit (CDC): COVID-19 vaccine information found in addendum
- Multilingual COVID-19 Resources (FDA)
- General Best Practice Guidelines for Immunization
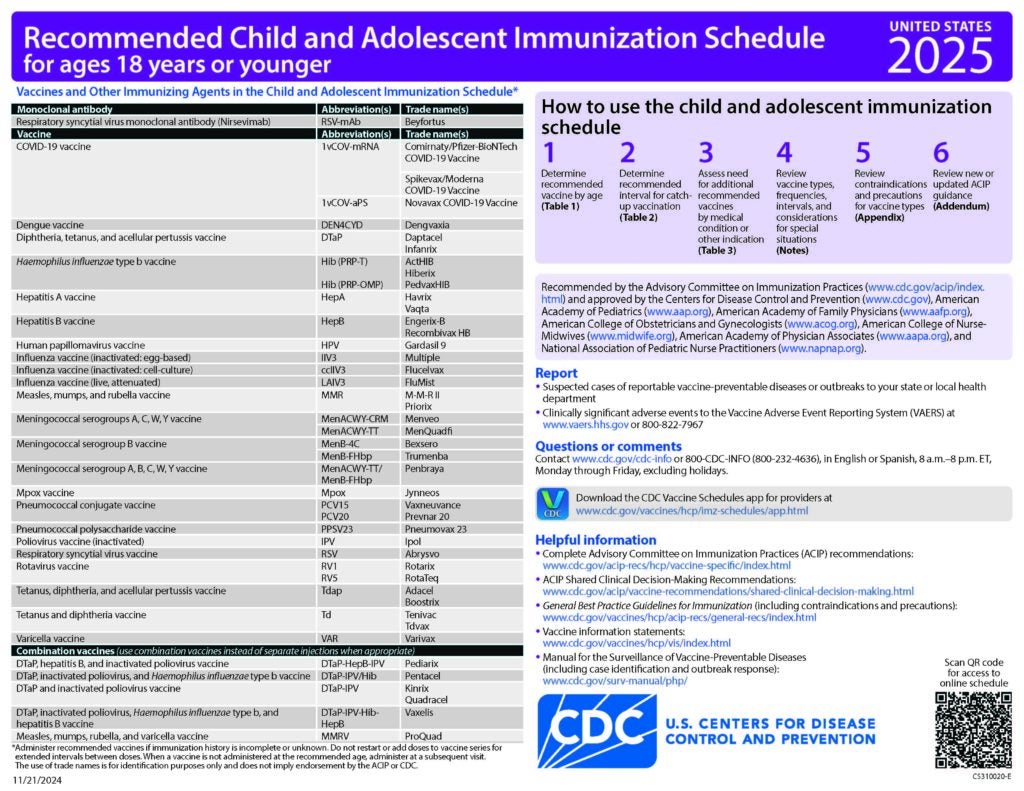
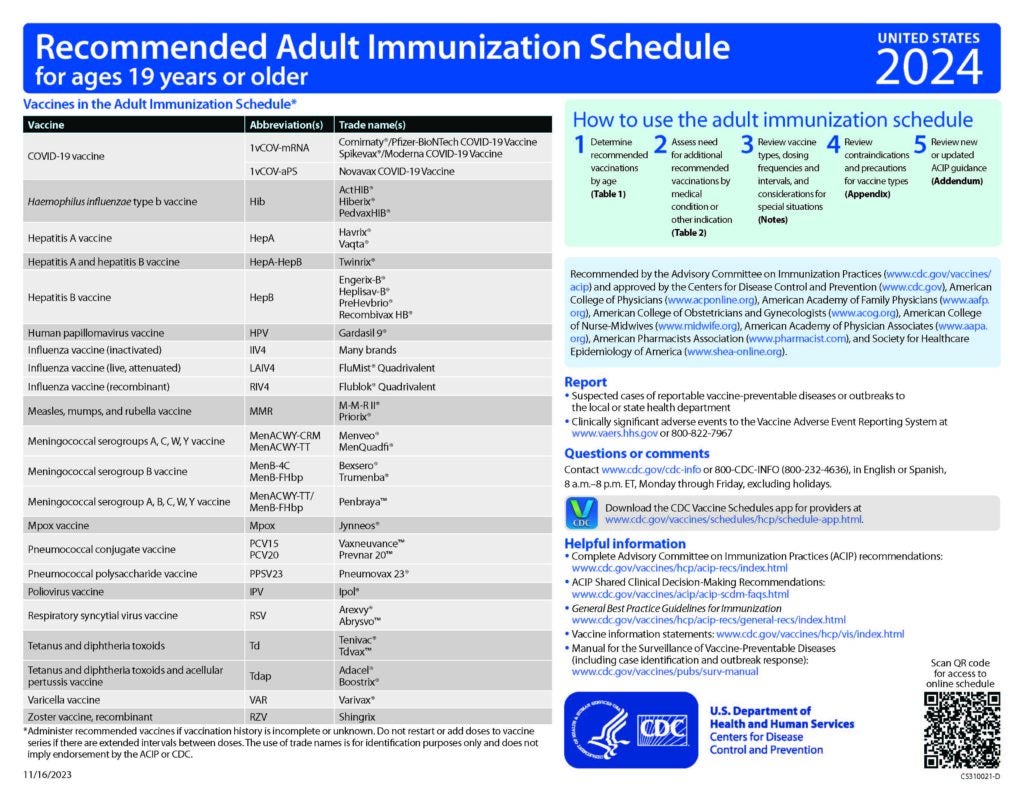
CDC Recommended Schedules
Source: The Morbidity and Mortality Weekly Report (MMWR) series prepared by CDC.
Source: The Morbidity and Mortality Weekly Report (MMWR) series prepared by CDC.